Selection, Preparation and Application of Graphene Raw Materials
Foreword
In 2004, the Geim team at the University of Manchester first obtained a single or thin layer of a new two-dimensional atomic crystal, graphene, by mechanical exfoliation. The discovery of graphene enriched the carbon family and formed a complete system from zero-dimensional fullerenes, one-dimensional CNTs, two-dimensional graphene to three-dimensional diamond and graphite. Graphene is the thinnest two-dimensional material found so far. It can be bent into zero-dimensional fullerenes, curled into one-dimensional CNTs, or stacked into three-dimensional graphite. This unique structure contains rich and peculiar physical phenomena, making graphene exhibit many excellent physicochemical properties with amazing electronic and mechanical characteristics. These properties make it suitable for a wide range of applications. Based on previous research, this paper mainly introduces the purification of graphite, the selection of raw materials for graphene preparation, the methods of graphene preparation, and its application.
Chapter 1 Graphite Purification
Naturally occurring graphite is rarely pure, often containing 10% to 20% impurities, including SiO2, Al2O3, MgO, CaO, P2O5, CuO, V2O5, H2O, S, FeO, and H, N, CO2, CH4, NH3, etc. Graphite minerals are iron black, steel gray, with a black streak; they have a metallic luster, and cryptocrystalline aggregates are dull and opaque; they are soft, with a density of 2.09–2.23 g/cm³. Currently, various methods for purifying graphite at home and abroad mainly include flotation, alkali acid, hydrofluoric acid, chlorination roasting, and high-temperature purification, each with their own advantages and disadvantages.
1.1 Flotation Method
The flotation method is a commonly used technique for mineral purification. Due to the poor wettability of graphite surfaces by water, it has good floatability and can be easily separated from impurity minerals. In China, flotation is generally used for graphite ore dressing. The flotation process usually involves positive flotation followed by reverse flotation of the concentrate. A higher-grade graphite concentrate can be obtained through flotation. Flotation graphite concentrates typically reach 80–90% grade, and with multi-stage grinding, purity can reach 98%. However, some impurities are embedded in the graphite scales in fine granular form, and even after fine grinding, they cannot be fully dissociated. Therefore, it is difficult to completely remove them using physical beneficiation methods. This part of the impurities is generally only used as the first step in graphite purification, and further purification is usually done via chemical or high-temperature methods.
Example:
(1) Mountain Southeast Villa Graphite Mine: Located in Laixi County, Shandong Province, producing flake graphite. The processing capacity of the ore dressing plant is about 350,000 t/a. The original ore size is -450 mm, the grinding particle size is -15 mm, and the selected particle size is -0.15 mm, accounting for 60–65%. The ore grade is 4.39–4.45%, the concentrate grade is 88–89%, the recovery rate is around 80%, and the tailings grade is 0.6–0.8%. After purification by the alkali acid method, the final graphite concentrate grade reaches 98–99%, with an operation recovery rate of 88%.
(2) Inner Mongolia Xinghe Graphite Mine: Two plants have an annual processing capacity of 300,000 tons of raw ore, with a graphite output of about 7,000 tons. The original ore size is -350 mm, the grinding particle size is -10 mm, and the selected particle size is -0.15 mm, accounting for 38%. The ore grade is 3.82–4.07%, the concentrate grade is about 88%, the tailings grade is 1.34–1.68%, and the recovery rate is about 68%. Flotation concentrates can be chemically purified to a grade of 94–99.5%.
(3) Heilongjiang Liumao Graphite Mine: Produces flake graphite. The original ore size is -550 mm, the grinding particle size is -20 mm, the selected particle size is -100 mesh, 70%. The original ore grade is 14–16%, and after five to six re-grindings, the selected and medium concentrates are returned to the rough grinding circuit. The refined ore grade is 93–95%, the tailings grade is 3–4%, and the ore recovery rate is about 75%.
(4) Hunan Lutang Graphite Mine: Produces cryptocrystalline graphite. The original ore grade is 65–68%, and the particle size is -250 mm, which is fine-grained. The processing flow is relatively simple, consisting mainly of processes such as hand selection, coarse crushing, screening, medium crushing, drying, grinding, and classification. The ore processing capacity of the ore dressing plant is about 60,000 t/d, and the grinding particle size is -40 mm. Hand-selected concentrate grades are 70–88%, and the tailings grade is less than 60%, with a beneficiation recovery rate of 90%.
1.2 Alkali Acid Method
The alkali acid method is the main method for chemical purification of graphite and is also a relatively mature process. It includes systems such as NaOH-HCl, NaOH-H2SO4, and NaOH-HCl-HNO3. Among these, the NaOH-HCl method is the most common. From current literature, the purity of high-purity graphite can reach 99%, but it does not meet the requirement of 99.9%. The acid-base method is widely used in China's high-purity graphite manufacturers. It has the advantages of low investment, high product quality, and strong adaptability. It also has the advantages of easy equipment realization and versatility. However, it requires high-temperature sintering, melting, large energy consumption, long reaction time, severe equipment corrosion, large graphite loss, and serious wastewater pollution.
1.3 Hydrofluoric Acid Method
When the mica content of the treated graphite is high, the effect of using the alkali acid method is not very good. At this point, the main advantage of the hydrofluoric acid method is its high impurity removal efficiency, high-quality products, and minimal impact on performance with low energy consumption. It can increase the fixed carbon content of graphite to 99.95%. However, the disadvantage is that hydrofluoric acid is highly toxic and corrosive. Strict safety measures must be taken during production, and environmental protection investments significantly increase the cost of the hydrofluoric acid method.
1.4 Chlorination Roasting Method
The chlorination roasting method involves adding a certain amount of reducing agent to the graphite powder, calcining it at a specific temperature and atmosphere, and then passing chlorine gas to carry out a chemical reaction. This allows valuable metals in the material to be converted into a gas phase or condensed phase with a lower melting point. The chloride and complex escape and separate from the remaining components to achieve graphite purification. This method has advantages such as energy saving, high purification efficiency (up to 98%), and high recovery rate. However, the toxicity, severe corrosion, and serious environmental pollution caused by chlorine gas limit the promotion and application of the chlorination roasting process. Additionally, the process is difficult to produce ultra-pure graphite, and the process system is not stable enough, which affects its application in actual production.
1.5 High-Temperature Purification Method
Graphite is one of the most heat-resistant substances in nature, with a melting point of 3,850 ± 50°C and a boiling point of 4,500°C. The boiling point of silicate minerals is below 2,750°C (the boiling point of quartz). The boiling point of graphite is much higher. This property is the theoretical basis for the purification of graphite by high-temperature methods. The high-temperature method can produce more than 99.99% ultra-high-purity graphite, but it requires the raw material's fixed carbon content to be over 99%, expensive equipment, huge investment, limited production scale, strict electric furnace heating technology, and the need to isolate air. Otherwise, when heated to 450°C in hot air, graphite begins to oxidize. The higher the temperature, the greater the loss of graphite. Only in special industries (such as national defense, aerospace, etc.) where the quality of graphite is very high, high-purity graphite is produced in small batches by the high-temperature method.
Chapter II Selection of Graphene Materials
In the process of preparing graphene from natural graphite, how to obtain single-layer graphene with higher purity becomes an important problem. For the selection of natural graphite for the preparation of graphene raw materials, the Hummers method was used to prepare graphene comparative tests through crystalline graphite and earthy graphite, and the following conclusions were drawn:
(1) For the preparation of graphene with larger particle size and higher purity, the raw material selection is preferably flake graphite, with the best particle size between 200–300 mesh, but not less than 300 mesh. If the particle size is too large, it affects the sufficiency of the reaction in the process, leading to a decrease in graphene purity, yield, and an increase in defect degree. If the particle size is too small, it leads to smaller particle size and increased defect degree of the prepared graphene.
(2) Graphene prepared from crystalline graphite as a raw material has lower resistivity and structural defect content compared to graphene prepared from microcrystalline graphite.
(3) Under the same oxidation-thermal stripping reduction conditions, the supercapacitor properties of graphene materials prepared from crystalline graphite are slightly better than those of graphene prepared from microcrystalline graphite.
(4) Under the same temperature conditions, the graphene prepared by crystalline graphite has less adsorption than the graphene prepared by microcrystalline graphite.
The characterization methods of graphene mainly include Raman spectroscopy, ultraviolet spectroscopy, XRD, SEM, etc. Raman spectroscopy is common, mainly used to reflect the defect density of graphene. The lower the defect density, the higher the crystallization degree, the better. The more highly ordered the olefin, the more sparse the graphite layer is directly connected to the layer, and the higher purity graphene is easily obtained. On the contrary, the tighter it is, it is not easy to obtain graphene with higher purity.
For highly ordered graphite, two peaks generally appear on the Raman spectrum, around 1350 cmâ»Â¹ (called D band) and around 1580 cmâ»Â¹ (called G band), and the G band is composed of two Eâ‚‚g. The Raman active vibration mode is generated, the peak shape is narrower and the intensity is higher, indicating the vibration of sp² hybridized carbon in the graphite structure; in general, the appearance of the D band indicates that the graphite has edges, defects or irregular carbon, etc. Due to the high degree of order of graphite, the strength of the belt is very weak. For this reason, we can use the ratio of the intensity of the D-band to the intensity of the G-band as a measure of the degree of disorder in the carbon material structure, i.e., the defect density, which is (ID/IG). The larger the ID/IG, the greater the disadvantage.
(1) The Raman shift is a defect peak at 1350 cmâ»Â¹, which is compared with the intrinsic peak. If the defect density is high, the peak value may be equal to the peak height at 1580 cmâ»Â¹;
(2) The Raman shift is 10.8 cmâ»Â¹, which is the highest peak. It can also be seen in its full width at half maximum, and its full width at half maximum is also widened as the density of defects increases;
(3) The Raman shift is 2720 cmâ»Â¹, and its peak height is about 1/2~1/3 of 1580.
Chapter III Preparation of Graphene
Currently, the synthesis methods of graphene mainly include mechanical exfoliation, surface epitaxy of silicon carbide, epitaxial growth, chemical vapor deposition, chemical dispersion, and chemical synthesis. Among them, the mechanical exfoliation and chemical dispersion methods are based on natural flake graphite with high purity (more than 90%), and the chemical dispersion method is currently recognized as a method for mass production.
2.1 Mechanical Peeling Method
This method first uses an ion beam to perform ion etching on a 1 mm thick highly oriented pyrolytic graphite surface by dry etching with oxygen plasma. A micro groove with a width of 2 μm ~ 2 mm and a depth of 5 μm is etched on the surface, and is adhered to the glass substrate by photoresist; then repeatedly peeled off with transparent tape to remove excess highly oriented pyrolytic graphite HOPG (highly oriented pyrolytic graphite); the glass substrate to which the microchip is adhered is then placed in an acetone solution for sonication; and the single crystal silicon wafer is placed in an acetone solvent to remove the single layer of graphene. Single-layer graphene is adsorbed on the single crystal silicon wafer due to van der Waals force or capillary force. Using this method, a quasi-two-dimensional graphite monolayer was successfully prepared and its morphology was observed. A silicon wafer containing a single layer of graphene prepared by micromechanical stripping was placed on an etched metal frame, and the silicon wafer was etched away with an acid to obtain a suspended single-layer graphene supported by a metal support. The morphology was observed by transmission electron microscopy and it was found that the single-layer graphene was not a flat plane but a wrinkle with a certain height (50 Å ~ 100 Å) above the plane. Through the study of the degree of wrinkling on the surface of single-layer graphene and double-layer graphene, it is found that the wrinkles on the surface of graphene may be a necessary condition for the existence of two-dimensional graphene. The surface wrinkles of single-layer graphene are significantly larger than that of bi-layer graphene, and the wrinkles become smaller and smaller as the number of graphene layers increases. This is because the single-layer graphene sheet is converted from two-dimensional to three-dimensional topography in order to reduce its surface energy. Although it is difficult to prepare graphene on a large scale by this method, and the size is not easy to control, the mechanical exfoliation method is still one of the most effective methods for preparing high-quality graphene.
2.2 Chemical Dispersion Method
There are three main methods for preparing water-soluble graphene oxide (GO) by chemical methods: Brodie, Staudenmaier, and Hummers. Either way, graphite is combined with a strong acid and a strong oxidizing agent, and a large amount of -OH, -COOH, and an epoxy group are introduced between the original CC skeleton of graphite. The C atom on the graphene oxide belongs to sp³ hybridization, which greatly destroys the planar structure of graphene, thereby reducing the excellent electrical conductivity of graphene. Therefore, many scientists are trying to reduce the graphite oxide by means of thermal annealing or chemical reduction to restore the original excellent performance. Graphene oxide is currently the most studied class of graphene derivatives with good solubility in water, ethylene glycol, DMF, NMP, and THF. With the in-depth development of the preparation methods, some scientists have revised or developed the original high-quality graphene method for chemical preparation of solution processing.
As a commonly used method, the Hummers method for preparing graphene is as follows: 1. First, the raw graphite is treated with an inorganic strong protonic acid (such as concentrated nitric acid, fuming nitric acid, or a mixture thereof) for inserting a strong acid small molecule into the graphite layer; 2. Oxidize it with a strong oxidizing agent (such as KMnO4, KClO4) to form a graphite oxide containing carboxyl groups and hydroxyl groups at the edges and containing oxygen groups such as epoxy and carbonyl groups; this process can make the distance between graphite layers from 0.34 nm expanded to 0.78 nm; 3. Further exfoliated by external force (ultrasonic stripping) to obtain a graphene oxide having a single atomic degree; 4. Further reduction can produce graphite.
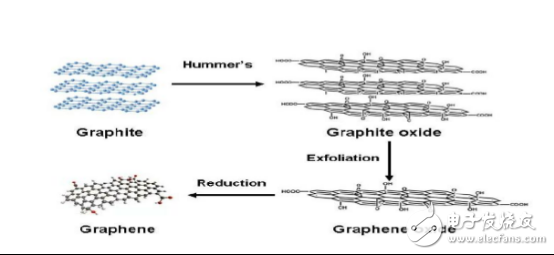
Preparation of graphene process diagram
2.3 Surface Epitaxy of Silicon Carbide
The method removes silicon by heating single crystal SiC, and decomposes the graphene sheet on the single crystal (001) surface [11] (in ultra-high vacuum, 1000 °C, silicon will be released, and the only remaining graphitized carbon). This method can controllably produce single-layer or multi-layer graphene (up to 100 layers of multi-layer graphene), the thickness of which is determined by the heating temperature, the disadvantage is the preparation of a large area, a single thickness of graphene more difficult. The specific method is: the sample obtained by etching with oxygen or hydrogen is heated by electron bombardment under high vacuum to remove oxides. After the Auger electron spectroscopy is used to confirm that the oxide on the surface is completely removed, the sample is heated to a temperature of 1250 °C to 1450 °C, and the temperature is maintained for 1 minute to 20 minutes to obtain a very thin graphene layer. DeHeer et al. [12-14] of the California Institute of Technology successfully used graphene to prepare graphene. However, the two-dimensional graphite prepared by this method did not observe the quantum exhibited by the two-dimensional graphite peeled off by HOPG. The Hall effect, and the electronic properties of the graphene surface are greatly affected by the SiC substrate, further research is still in progress.
2.4 Orientation Episode
The method utilizes the growth matrix atomic structure to "grow" graphene. The carbon atoms are infiltrated into the metal crucible at a temperature of 1150 °C, and then cooled to 850 °C, at which a large number of carbon atoms float up to the surface of the crucible, and a sheet-like monolayer of carbon atoms "islands" are formed on the surface of the substrate. Finally, grow into a complete layer of graphene. After the first layer covers 80% of the substrate, the second layer begins to grow. The underlying graphene will have a strong interaction with the ruthenium, while the second layer will be almost completely separated from the ruthenium, leaving only weakly coupled, resulting in a satisfactory single-layer graphene sheet. A disadvantage of this method is that the resulting graphene sheets tend to be non-uniform in thickness, and the adhesion between the graphene and the matrix affects the properties of the carbon layer.
2.5 Chemical Vapor Deposition
Chemical vapor deposition (CVD) is the most widely used method for large-scale industrial preparation of semiconductor thin film materials, and has become a way for researchers to prepare graphene. So far, the way to prepare graphene by chemical deposition has been further explored and improved. At present, the immaturity of the process and the high cost have limited its large-scale application. How to produce high-quality graphene materials in large quantities and at low cost should be a focus of future research.
2.6 Chemical Synthesis
Mainly using benzene ring compounds as raw materials to synthesize graphene. In 2008, the Muellen team used organic synthesis to obtain graphene compounds with a thickness of 12 nm. Although the electrical properties of this material have not been determined, it should undoubtedly have properties similar to those of graphene. The chemical synthesis method can produce continuous and excellent graphene thin film semiconductor materials, and the existing semiconductor processing technology can also modify the graphene film material, so that the graphene material prepared by the chemical growth method has microelectronics field. Great application potential. If the organic synthesis method can break through the problem of the smaller size of the produced graphene in the next few years, it will provide a broad prospect for the application of graphene.
Chapter IV Functionalization of Graphene
The so-called functionalization is to use the defects and groups of the graphene during the preparation process to change some properties of the graphene surface by covalent, non-covalent or doping methods, which is easier to study and apply.
The problem that graphene will face in the future is how to achieve its controllable functionalization. The structurally complete graphene is a two-dimensional crystal composed of a benzene six-membered ring containing no unstable bonds. It has high chemical stability, its surface is inert, and its interaction with other media (such as solvents) is weak. And there is a strong van der Waals force between the graphene sheet and the sheet, which tends to cause aggregation, making it difficult to dissolve in water and common organic solvents, which causes great difficulty for further research and application of graphene, in order to fully utilize graphene, it must be effectively functionalized by exerting its excellent properties and improving its moldability (such as improving solubility, dispersibility in a matrix, etc.). By introducing specific functional groups, it is also possible to impart new properties to graphene and further expand its application fields. Functionalization is the most important means of achieving graphene dispersion, dissolution and molding. The discovery of graphene two-dimensional crystals has opened an exciting page for condensed matter physics research, and the functionalization and application of graphene will provide new opportunities in the fields of chemistry and materials.
Chapter 5 Graphene Applications
Graphene is currently the thinnest (the theoretical thickness of only 0.35 nm) but the hardest nanomaterial (more than 100 times the strength of steel, reaching 130 GPa), almost completely transparent, only absorbing 2.3% of light; the world's smallest resistivity material, because of its extremely low resistivity and extremely fast speeds, is expected to be used to develop a new generation of electronic components or transistors that are thinner and more electrically conductive; since graphene is essentially a transparent, good conductor, also suitable for the manufacture of transparent touch screens, light panels, and even solar cells; excellent thermal conductivity (thermal conductivity is three times that of diamond, reaching 5,000 W·mâ»Â¹Â·Kâ»Â¹), zero band gap, high electron/hole mobility (in theory, up to 200,000 cm²·Vâ»Â¹Â·sâ»Â¹, which is twice as high as the currently known indium telluride material with the highest mobility, exceeding commercial silicon sheet 10 times the mobility of the sheet; at normal temperature, even if the surrounding carbon atoms collide, the electrons in the graphene are very little interfered. Graphene also exhibits integer and fractional quantum Hall effects and room temperature ferromagnetism at room temperature. The unique structure of graphene and its excellent electrical, optical, thermal and mechanical properties have attracted the attention of countless physicists, chemists and materials scientists, and have opened the graphene era.
5.1 Polymer Composites
Graphene has excellent properties and low cost. After functionalization, graphene can be processed by conventional methods such as solution processing, which is very suitable for developing high-performance polymer composite materials. Ruoff and others are indicators of graphene-polystyrene. Conductive materials have a conductive critical content of only 0.1%, which also greatly improves the thermal stability of the polymer. Chen et al. prepared a composite material of sulfonic acid group and isocyanate functionalized graphene and thermoplastic polyurethane (TPU), and studied the application of this material in infrared light trigger driving dear.
5.2 Photoelectric Functional Materials and Devices
The development of new optoelectronic functional materials and devices has greatly promoted the development of electronics, information and communication. Among them, nonlinear optical materials have important application prospects in image processing, optical switching, optical storage, and personnel and device protection. Good nonlinear optical materials usually have large dipole moments and π systems, and the structural characteristics of graphene meet these requirements.
5.3 Application in the Field of Information Storage
Compared with silicon memory, memory based on organic polymer storage materials has the advantages of low cost, easy processing, good flexibility, large area production, fast response, low power consumption, high density storage, etc., in information storage and high-speed computing. The field has a very broad application prospect.
5.4 Applications in Nanoelectronic Devices
At room temperature, the carrier mobility of graphene is ten times that of ordinary silicon wafers, and it is little affected by temperature and doping effect, showing the ballistic transmission characteristics of room temperature submicron scale. This is the most prominent advantage of graphene as a nanoelectronic device. In order to impart a certain electrical property to a single layer of graphene, graphene is cut according to a specific pattern to form a graphene nanoribbon. The structure of graphene nanoribbons has high electrical conductivity, high thermal conductivity, and low noise. These excellent qualities make graphene nanoribbons an alternative to integrated circuit interconnect materials, and may replace copper metal.
5.5 Graphene in Biological Applications
Due to its modified chemical function, large contact area, atomic size and thickness, and molecular gate structure, graphene is an excellent choice for bacterial detection and diagnostic devices. Graphene embedded in the biosensor interface can increase the effective surface area of the electrode and can be used as a support for metal nanoparticles.
5.6 Solar Cells
Indium tin oxide (ITO) has been widely used as an electrode material for solar cells due to its high electrical conductivity and light transmittance. However, due to the scarcity of indium resources, there is an urgent need to find alternatives to replace ITO. Graphene has good light penetration and electrical conductivity, and has the potential to be an alternative to ITO. The use of graphene to make transparent conductive films and their application in solar cells has also become a research hotspot.
5.7 Catalysts and Drug Carriers
Graphitized carbon materials, including graphite, carbon black, activated carbon, CNTs, carbon nanofibers, etc., have been widely used as catalyst carriers. A large number of studies have shown that the structure of the carbon support has a great influence on the performance of the supported catalyst; Graphene has a regular two-dimensional surface structure, which can be used as an ideal template-supporting catalyst. Graphene-based catalysts have higher catalytic activity. Since graphene has a monoatomic layer structure, its specific surface area is large, and because of its good biocompatibility, it is very suitable as a pharmaceutical carrier.
5.8 Gas Sensor and Single Molecule Gas Detection
Some important properties of graphene make it have a good development prospect in the manufacture and application of sensors. For example, the unique two-dimensional layered structure of graphene has a large specific surface area, which is a necessary factor for making high sensitivity sensors. In fact, this is another important reason why other nanostructured materials are used as sensors. Another important reason why graphene is used as a sensor is its unique electronic structure. The adsorption of certain gas molecules can induce changes in the electronic structure of graphene. As a result, its electrical conductivity rapidly changes greatly, even if a gas molecule is adsorbed or released.
Unlike silicon and metal materials used in current electronic devices, graphene also maintains good stability and electrical properties when reduced to nanoscale or even a single benzene ring, making it possible to explore single-electron devices.
5.9 Capacitor Equipment
New chemical power systems, especially secondary batteries and supercapacitors, are currently important "green" energy storage devices. Various carbon materials, such as amorphous carbon, porous carbon, graphite, etc., have been widely used in lithium ion batteries. Since graphene has a large specific surface area, a layered structure, a small size effect, and good catalytic activity, it can increase the specific capacity of the battery and can be an electrode material for an important energy storage device.
Energy storage component charging speed service life
| Energy storage component | Charging speed | Service life (times) |
Power density (W/kg) |
Energy Density (Wh/kg) |
| Lead-acid batteries NiMH batteries Lithium Ion Battery Activated carbon supercapacitor Graphene supercapacitor |
2hours 2hours 2hours 10seconds- 10minutes 10seconds- 10minutes |
300-1000 500-1000 ~1000 5000-10000 10000-50000 |
300 50-100 100-150 300-2000 300-5000 |
20-30 30-80 50-120 5-8 20-30 |
5.10 Antibacterial Substances
Scientists at the Shanghai Branch of the Chinese Academy of Sciences have found that graphene oxide is extremely effective in inhibiting the growth of E. coli and does not harm human cells. If graphene oxide is also antibacterial to other bacteria, it may find a range of new applications, such as shoes that automatically remove odors, or preserve fresh packaging of food.
5.11 Graphene Photosensitive Element
A group of scientists from Singapore specializing in graphene materials research and development of the latest technology to apply graphene to camera photosensitive elements, is expected to completely subvert the future development of digital sensor technology.
5.12 Thermally Conductive Materials and Thermal Interface Materials
When the graphene molecule contains 30% epoxy groups, it will have a very interesting property and can be used as a thermal interface material for some electronic instrument components and more advanced electronic devices. In 2011, the Georgia Institute of Technology scholars first reported the application of the three-dimensional structure of vertically aligned functionalized multilayer graphene in thermal interface materials and its ultra-high equivalent thermal conductivity and ultra-low interfacial thermal resistance.
5.13 Desalination of Seawater
Studies have shown that graphene filters may outperform other desalination technologies.
5.14 Transparent Conductive Electrode
The good electrical conductivity and light transmission properties of graphene make it a very good application prospect in transparent conducting electrodes. Touch screens, liquid crystal displays, organic photovoltaic cells, organic light emitting diodes, etc. all require good transparent conductive electrode materials. In particular, graphene is superior in mechanical strength and flexibility to indium tin oxide, a commonly used material. Due to the high brittleness of indium tin oxide, it is relatively easy to damage. The graphene film in solution can be deposited in a large area.
Conclusion and Outlook
Since the first discovery of graphene in 2004, significant progress has been made in the preparation of graphene and the application of graphite. However, there are still many problems in how to prepare higher purity graphene and industrial production. The preparation of graphene and its functional materials are still the general direction of future development.
High-purity graphite refers to the carbon content of graphite “99.9-99.99%â€, which is widely used in advanced refractory materials and coatings for metallurgical industry, stabilizer for military industrial pyrotechnic materials, pencil core for light industry, carbon brush for electric industry, battery industry electrode, catalyst additives for fertilizer industry, etc.
High carbon graphite refers to carbon content (≥) 94.00-99.00
Medium carbon graphite refers to carbon content (≥) 80.00-93.00, medium carbon graphite is mainly used in the production of foundry coatings, refractory materials, etc.
Graphite market
In December 2015, the northeast region-195 flake graphite mainstream factory tax-included price was 2800-2900 yuan / ton, -194 flake graphite mainstream factory tax-included price was 2700-2800 yuan / ton, the price was lowered by 100 yuan / ton. Shandong area scale graphite-195 mainstream factory tax price is 3200-3400 yuan / ton, -194 factory tax-included price is 3000-3200 yuan / ton, the price is lowered by 100 yuan / ton.
2015-12-31 China's Henan area flake graphite - 190 reported 2200 yuan / ton; Northeast -190 reported 2,000 yuan / ton, -195 reported 2800-3000 yuan / ton; Shandong area -195 reported 3200 yuan / ton Left and right, 895 reported 5200-5400 yuan / ton, 195 reported 4800 yuan / ton, -199 reported 5700-6300 yuan / ton; Inner Mongolia 595 reported 9700 yuan / ton, 895 reported 5500-6000 yuan / ton, - 195 reported 3700 yuan / ton.
+100
-100
-200
-325
Medium carbon graphite:
Medium carbon graphite refers to carbon content (≥) 80.00-93.00, medium carbon graphite is mainly used in the production of foundry coatings, refractory materials and so on.
Technical indicators:
| Brand | Technical indicators | The main purpose | ||
| Fixed carbon (≥%) | Moisture (≤%) | Screening allowance (%) | ||
